Single-cell transcriptomic analysis of somatosensory neurons uncovers temporal development of neuropathic pain
- PMID: 33692491
- PMCID: PMC8324866
- DOI: 10.1038/s41422-021-00479-9
Single-cell transcriptomic analysis of somatosensory neurons uncovers temporal development of neuropathic pain
Erratum in
-
Publisher Correction: Single-cell transcriptomic analysis of somatosensory neurons uncovers temporal development of neuropathic pain.Cell Res. 2021 Aug;31(8):939-940. doi: 10.1038/s41422-021-00503-y. Cell Res. 2021. PMID: 34012074 Free PMC article. No abstract available.
Abstract
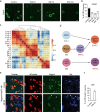
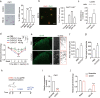
Peripheral nerve injury could lead to chronic neuropathic pain. Understanding transcriptional changes induced by nerve injury could provide fundamental insights into the complex pathogenesis of neuropathic pain. Gene expression profiles of dorsal root ganglia (DRG) in neuropathic pain condition have been studied. However, little is known about transcriptomic changes in individual DRG neurons after peripheral nerve injury. Here we performed single-cell RNA sequencing on dissociated mouse DRG cells after spared nerve injury (SNI). In addition to DRG neuron types that are found under physiological conditions, we identified three SNI-induced neuronal clusters (SNIICs) characterized by the expression of Atf3/Gfra3/Gal (SNIIC1), Atf3/Mrgprd (SNIIC2) and Atf3/S100b/Gal (SNIIC3). These SNIICs originated from Cldn9+/Gal+, Mrgprd+ and Trappc3l+ DRG neurons, respectively. Interestingly, SNIIC2 switched to SNIIC1 by increasing Gal and reducing Mrgprd expression 2 days after nerve injury. Inferring the gene regulatory networks after nerve injury, we revealed that activated transcription factors Atf3 and Egr1 in SNIICs could enhance Gal expression while activated Cpeb1 in SNIIC2 might suppress Mrgprd expression within 2 days after SNI. Furthermore, we mined the transcriptomic changes in the development of neuropathic pain to identify potential analgesic targets. We revealed that cardiotrophin-like cytokine factor 1, which activates astrocytes in the dorsal horn of spinal cord, was upregulated in SNIIC1 neurons and contributed to SNI-induced mechanical allodynia. Therefore, our results provide a new landscape to understand the dynamic course of neuron type changes and their underlying molecular mechanisms during the development of neuropathic pain.
© 2021. The Author(s), under exclusive licence to Center for Excellence in Molecular Cell Science, CAS.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
A Transcriptomic Analysis of Neuropathic Pain in Rat Dorsal Root Ganglia Following Peripheral Nerve Injury.Neuromolecular Med. 2020 Jun;22(2):250-263. doi: 10.1007/s12017-019-08581-3. Epub 2019 Dec 19. Neuromolecular Med. 2020. PMID: 31858405
-
Upregulation of Chemokine CXCL12 in the Dorsal Root Ganglia and Spinal Cord Contributes to the Development and Maintenance of Neuropathic Pain Following Spared Nerve Injury in Rats.Neurosci Bull. 2016 Feb;32(1):27-40. doi: 10.1007/s12264-015-0007-4. Epub 2016 Jan 19. Neurosci Bull. 2016. PMID: 26781879 Free PMC article.
-
Sigma-1 receptors control neuropathic pain and macrophage infiltration into the dorsal root ganglion after peripheral nerve injury.FASEB J. 2020 Apr;34(4):5951-5966. doi: 10.1096/fj.201901921R. Epub 2020 Mar 10. FASEB J. 2020. PMID: 32157739
-
Role of the immune system in neuropathic pain.Scand J Pain. 2019 Dec 18;20(1):33-37. doi: 10.1515/sjpain-2019-0138. Scand J Pain. 2019. PMID: 31730538 Review.
-
[Contribution of primary sensory neurons and spinal glial cells to pathomechanisms of neuropathic pain].Brain Nerve. 2008 May;60(5):483-92. Brain Nerve. 2008. PMID: 18516970 Review. Japanese.
Cited by
-
Optogenetic Activation of Peripheral Somatosensory Neurons in Transgenic Mice as a Neuropathic Pain Model for Assessing the Therapeutic Efficacy of Analgesics.ACS Pharmacol Transl Sci. 2023 Dec 13;7(1):236-248. doi: 10.1021/acsptsci.3c00254. eCollection 2024 Jan 12. ACS Pharmacol Transl Sci. 2023. PMID: 38230281 Free PMC article.
-
A developmental atlas of somatosensory diversification and maturation in the dorsal root ganglia by single-cell mass cytometry.Nat Neurosci. 2022 Nov;25(11):1543-1558. doi: 10.1038/s41593-022-01181-8. Epub 2022 Oct 27. Nat Neurosci. 2022. PMID: 36303068 Free PMC article.
-
Single-cell transcriptome atlas of the human corpus cavernosum.Nat Commun. 2022 Jul 25;13(1):4302. doi: 10.1038/s41467-022-31950-9. Nat Commun. 2022. PMID: 35879305 Free PMC article.
-
Peripheral BDNF Regulates Somatosensory-Sympathetic Coupling in Brachial Plexus Avulsion-Induced Neuropathic Pain.Neurosci Bull. 2023 Dec;39(12):1789-1806. doi: 10.1007/s12264-023-01075-0. Epub 2023 Jun 19. Neurosci Bull. 2023. PMID: 37335428 Free PMC article.
-
Transcriptomic analysis of differentially alternative splicing patterns in mice with inflammatory and neuropathic pain.Mol Pain. 2024 Jan-Dec;20:17448069241249455. doi: 10.1177/17448069241249455. Mol Pain. 2024. PMID: 38597175 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

