Decreased Level of Exosomal miR-5121 Released from Microglia Suppresses Neurite Outgrowth and Synapse Recovery of Neurons Following Traumatic Brain Injury
- PMID: 33475953
- PMCID: PMC8423926
- DOI: 10.1007/s13311-020-00999-z
Decreased Level of Exosomal miR-5121 Released from Microglia Suppresses Neurite Outgrowth and Synapse Recovery of Neurons Following Traumatic Brain Injury
Abstract
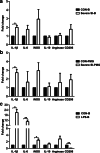
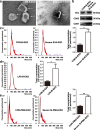
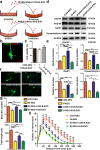
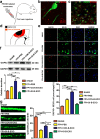
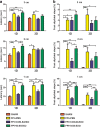
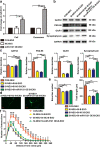
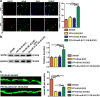
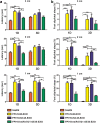
Activated microglia can suppress neurite outgrowth and synapse recovery in the acute stage following traumatic brain injury (TBI). However, the underlying mechanism has not been clearly elucidated. Exosomes derived from microglia have been reported to play a critical role in microglia-neuron interaction in healthy and pathological brains. Here, we aimed to investigate the role of microglia-derived exosomes in regulating neurite outgrowth and synapse recovery following TBI. In our study, exosomes derived from microglia were co-cultured with stretch-injured neurons in vitro and intravenously injected into mice that underwent fluid percussion injury (FPI) by tail vein injection in vivo. The results showed that microglia-derived exosomes could be absorbed by neurons in vitro and in vivo. Moreover, exosomes derived from stretch-injured microglia decreased the protein levels of GAP43, PSD-95, GluR1, and Synaptophysin and dendritic complexity in stretch-injured neurons in vitro, and reduced GAP43+ NEUN cell percentage and apical dendritic spine density in the pericontusion region in vivo. Motor coordination was also impaired in mice treated with stretch-injured microglia-derived exosomes after FPI. A microRNA microarray showed that the level of miR-5121 was decreased most greatly in exosomes derived from stretch-injured microglia. Overexpression of miR-5121 in stretch-injured microglia-derived exosomes partly reversed the suppression of neurite outgrowth and synapse recovery of neurons both in vitro and in vivo. Moreover, motor coordination in miR-5121 overexpressed exosomes treated mice was significantly improved after FPI. Following mechanistic study demonstrated that miR-5121 might promote neurite outgrowth and synapse recovery by directly targeting RGMa. In conclusion, our finding revealed a novel exosome-mediated mechanism of microglia-neuron interaction that suppressed neurite outgrowth and synapse recovery of neurons following TBI.
Keywords: Exosomes; microRNA; microglia; neuron; traumatic brain injury.
© 2021. The American Society for Experimental NeuroTherapeutics, Inc.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Increased level of exosomal miR-20b-5p derived from hypothermia-treated microglia promotes neurite outgrowth and synapse recovery after traumatic brain injury.Neurobiol Dis. 2023 Apr;179:106042. doi: 10.1016/j.nbd.2023.106042. Epub 2023 Feb 17. Neurobiol Dis. 2023. PMID: 36804284
-
Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons.FASEB J. 2018 Jan;32(1):512-528. doi: 10.1096/fj.201700673R. Epub 2017 Sep 21. FASEB J. 2018. PMID: 28935818
-
Increases in miR-124-3p in Microglial Exosomes Confer Neuroprotective Effects by Targeting FIP200-Mediated Neuronal Autophagy Following Traumatic Brain Injury.Neurochem Res. 2019 Aug;44(8):1903-1923. doi: 10.1007/s11064-019-02825-1. Epub 2019 Jun 12. Neurochem Res. 2019. PMID: 31190315
-
Exosomal microRNAs have great potential in the neurorestorative therapy for traumatic brain injury.Exp Neurol. 2022 Jun;352:114026. doi: 10.1016/j.expneurol.2022.114026. Epub 2022 Feb 25. Exp Neurol. 2022. PMID: 35227684 Review.
-
The Role of Microglial Exosomes and miR-124-3p in Neuroinflammation and Neuronal Repair after Traumatic Brain Injury.Life (Basel). 2023 Sep 16;13(9):1924. doi: 10.3390/life13091924. Life (Basel). 2023. PMID: 37763327 Free PMC article. Review.
Cited by
-
Differential Stimulation of Pluripotent Stem Cell-Derived Human Microglia Leads to Exosomal Proteomic Changes Affecting Neurons.Cells. 2021 Oct 24;10(11):2866. doi: 10.3390/cells10112866. Cells. 2021. PMID: 34831089 Free PMC article.
-
Efficacy of extracellular vesicles of different cell origins in traumatic brain injury: A systematic review and network meta-analysis.Front Neurosci. 2023 Mar 29;17:1147194. doi: 10.3389/fnins.2023.1147194. eCollection 2023. Front Neurosci. 2023. PMID: 37065922 Free PMC article.
-
Immunomodulatory effect of extracellular vesicles from Entamoeba histolytica trophozoites: Regulation of NETs and respiratory burst during confrontation with human neutrophils.Front Cell Infect Microbiol. 2022 Oct 28;12:1018314. doi: 10.3389/fcimb.2022.1018314. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36389143 Free PMC article.
-
Neuroprotective and Neurotoxic Effects of Glial-Derived Exosomes.Front Cell Neurosci. 2022 Jun 22;16:920686. doi: 10.3389/fncel.2022.920686. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35813501 Free PMC article. Review.
-
Therapeutics of Extracellular Vesicles in Cardiocerebrovascular and Metabolic Diseases.Adv Exp Med Biol. 2023;1418:187-205. doi: 10.1007/978-981-99-1443-2_13. Adv Exp Med Biol. 2023. PMID: 37603281
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

