Bioglass could increase cell membrane fluidity with ion products to develop its bioactivity
- PMID: 33043500
- PMCID: PMC7653244
- DOI: 10.1111/cpr.12906
Bioglass could increase cell membrane fluidity with ion products to develop its bioactivity
Abstract
Objectives: Silicate bioactive glass (BG) has been widely demonstrated to stimulate both of the hard and soft tissue regeneration, in which ion products released from BG play important roles. However, the mechanism by which ion products act on cells on cells is unclear.
Materials and methods: Human umbilical vein endothelial cells and human bone marrow stromal cells were used in this study. Fluorescence recovery after photobleaching and generalized polarization was used to characterize changes in cell membrane fluidity. Migration, differentiation and apoptosis experiments were carried out. RNA and protein chip were detected. The signal cascade is simulated to evaluate the effect of increased cell membrane fluidity on signal transduction.
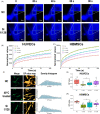
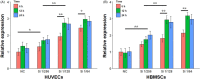
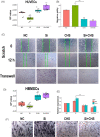
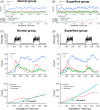
Results: We have demonstrated that ion products released from BG could effectively enhance cell membrane fluidity in a direct and physical way, and Si ions may play a major role. Bioactivities of BG ion products on cells, such as migration and differentiation, were regulated by membrane fluidity. Furthermore, we have proved that BG ion products could promote apoptosis of injured cells based on our conclusion that BG ion products increased membrane fluidity.
Conclusions: This study proved that BG ion products could develop its bioactivity on cells by directly enhancing cell membrane fluidity and subsequently affected cell behaviours, which may provide an explanation for the general bioactivities of silicate material.
Keywords: Bioglass; cell activity; membrane fluidity; silicon.
© 2020 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Bioglass/alginate composite hydrogel beads as cell carriers for bone regeneration.J Biomed Mater Res B Appl Biomater. 2014 Jan;102(1):42-51. doi: 10.1002/jbm.b.32978. Epub 2013 Jul 11. J Biomed Mater Res B Appl Biomater. 2014. PMID: 23847006
-
Bioglass enhanced wound healing ability of urine-derived stem cells through promoting paracrine effects between stem cells and recipient cells.J Tissue Eng Regen Med. 2018 Mar;12(3):e1609-e1622. doi: 10.1002/term.2587. Epub 2017 Nov 1. J Tissue Eng Regen Med. 2018. PMID: 29024443
-
Bioglass® 45S5-based composites for bone tissue engineering and functional applications.J Biomed Mater Res A. 2017 Nov;105(11):3197-3223. doi: 10.1002/jbm.a.36156. Epub 2017 Aug 9. J Biomed Mater Res A. 2017. PMID: 28686004 Review.
-
Vacuumed collagen-impregnated bioglass scaffolds: Characterization and influence on proliferation and differentiation of bone marrow stromal cells.J Biomed Mater Res B Appl Biomater. 2019 Feb;107(2):211-222. doi: 10.1002/jbm.b.34112. Epub 2018 Mar 23. J Biomed Mater Res B Appl Biomater. 2019. PMID: 29569333
-
A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics.Biomaterials. 2011 Apr;32(11):2757-74. doi: 10.1016/j.biomaterials.2011.01.004. Epub 2011 Feb 2. Biomaterials. 2011. PMID: 21292319 Review.
Cited by
-
Toxicological Assessment of Biodegradable Poli-ε-Caprolactone Polymer Composite Materials Containing Hydroxyapatite, Bioglass, and Chitosan as Potential Biomaterials for Bone Regeneration Scaffolds.Biomedicines. 2024 Aug 26;12(9):1949. doi: 10.3390/biomedicines12091949. Biomedicines. 2024. PMID: 39335462 Free PMC article.
-
Silica-Based Advanced Nanoparticles For Treating Ischemic Disease.Tissue Eng Regen Med. 2023 Apr;20(2):177-198. doi: 10.1007/s13770-022-00510-z. Epub 2023 Jan 23. Tissue Eng Regen Med. 2023. PMID: 36689072 Free PMC article. Review.
-
Bioactive glass selectively promotes cytotoxicity towards giant cell tumor of bone derived neoplastic stromal cells and induces MAPK signalling dependent autophagy.Bioact Mater. 2022 Feb 28;15:456-468. doi: 10.1016/j.bioactmat.2022.02.021. eCollection 2022 Sep. Bioact Mater. 2022. PMID: 35386334 Free PMC article.
-
Cobalt-Doped Bioactive Glasses for Biomedical Applications: A Review.Materials (Basel). 2023 Jul 14;16(14):4994. doi: 10.3390/ma16144994. Materials (Basel). 2023. PMID: 37512268 Free PMC article. Review.
-
Bioactive glasses incorporating less-common ions to improve biological and physical properties.J Mater Sci Mater Med. 2021 Dec 23;33(1):3. doi: 10.1007/s10856-021-06626-3. J Mater Sci Mater Med. 2021. PMID: 34940923 Free PMC article. Review.
References
-
- Yu H, Peng J, Xu Y, Chang J, Li H. Bioglass activated skin tissue engineering constructs for wound healing. ACS Appl Mater Interfaces. 2016;8(1):703‐715. - PubMed
-
- Fernandes JS, Gentile P, Pires RA, Reis RL, Hatton PV. Multifunctional bioactive glass and glass‐ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017;59:2‐11. - PubMed
-
- Dawood AE, Parashos P, Wong RHK, Reynolds EC, Manton DJ. Calcium silicate‐based cements: composition, properties, and clinical applications. J Investig Clin Dent. 2017;8(2):e12195. - PubMed
-
- Miguez‐Pacheco V, Hench LL, Boccaccini AR. Bioactive glasses beyond bone and teeth: emerging applications in contact with soft tissues. Acta Biomater. 2015;13:1‐15. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

