TfR1 binding with H-ferritin nanocarrier achieves prognostic diagnosis and enhances the therapeutic efficacy in clinical gastric cancer
- PMID: 32024821
- PMCID: PMC7002446
- DOI: 10.1038/s41419-020-2272-z
TfR1 binding with H-ferritin nanocarrier achieves prognostic diagnosis and enhances the therapeutic efficacy in clinical gastric cancer
Erratum in
-
Correction: TfR1 binding with H-ferritin nanocarrier achieves prognostic diagnosis and enhances the therapeutic efficacy in clinical gastric cancer.Cell Death Dis. 2022 Nov 25;13(11):998. doi: 10.1038/s41419-022-05453-w. Cell Death Dis. 2022. PMID: 36433933 Free PMC article. No abstract available.
Abstract
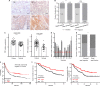
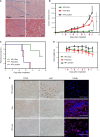
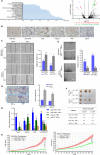
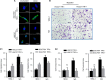
H-ferritin (HFn) nanocarrier is emerging as a promising theranostic platform for tumor diagnosis and therapy, which can specifically target tumor cells via binding transferrin receptor 1 (TfR1). This led us to investigate the therapeutic function of TfR1 in GC. The clinical significance of TfR1 was assessed in 178 GC tissues by using a magneto-HFn nanoparticle-based immunohistochemistry method. The therapeutic effects of doxorubicin-loaded HFn nanocarriers (HFn-Dox) were evaluated on TfR1-positive GC patient-derived xenograft (GC-PDX) models. The biological function of TfR1 was investigated through in vitro and in vivo assays. TfR1 was upregulated (73.03%) in GC tissues, and reversely correlated with patient outcome. TfR1-negative sorted cells exhibited tumor-initiating features, which enhanced tumor formation and migration/invasion, whereas TfR1-positive sorted cells showed significant proliferation ability. Knockout of TfR1 in GC cells also enhanced cell invasion. TfR1-deficient cells displayed immune escape by upregulating PD-L1, CXCL9, and CXCL10, when disposed with IFN-γ. Western blot results demonstrated that TfR1-knockout GC cells upregulated Akt and STAT3 signaling. Moreover, in TfR1-positive GC-PDX models, the HFn-Dox group significantly inhibited tumor growth, and increased mouse survival, compared with that of free-Dox group. TfR1 could be a potential prognostic and therapeutic biomarker for GC: (i) TfR1 reversely correlated with patient outcome, and its negative cells possessed tumor-aggressive features; (ii) TfR1-positive cells can be killed by HFn drug nanocarrier. Given the heterogeneity of GC, HFn drug nanocarrier combined with other therapies toward TfR1-negative cells (such as small molecules or immunotherapy) will be a new option for GC treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Optimized Doxorubicin Chemotherapy for Diffuse Large B-cell Lymphoma Exploits Nanocarrier Delivery to Transferrin Receptors.Cancer Res. 2021 Feb 1;81(3):763-775. doi: 10.1158/0008-5472.CAN-20-2674. Epub 2020 Nov 11. Cancer Res. 2021. PMID: 33177062 Free PMC article.
-
Endoscopic molecular imaging of early gastric cancer using fluorescently labeled human H-ferritin nanoparticle.Nanomedicine. 2018 Oct;14(7):2259-2270. doi: 10.1016/j.nano.2018.07.007. Epub 2018 Jul 26. Nanomedicine. 2018. PMID: 30056091
-
Combined Ferritin Nanocarriers with ICG for Effective Phototherapy Against Breast Cancer.Int J Nanomedicine. 2024 May 14;19:4263-4278. doi: 10.2147/IJN.S445334. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38766663 Free PMC article.
-
Ferritin: A Multifunctional Nanoplatform for Biological Detection, Imaging Diagnosis, and Drug Delivery.Acc Chem Res. 2021 Sep 7;54(17):3313-3325. doi: 10.1021/acs.accounts.1c00267. Epub 2021 Aug 20. Acc Chem Res. 2021. PMID: 34415728 Review.
-
Advancement of the study on iron metabolism and regulation in tumor cells.Chin J Cancer. 2010 Apr;29(4):451-5. doi: 10.5732/cjc.009.10716. Chin J Cancer. 2010. PMID: 20346225 Review.
Cited by
-
The dual role of FSP1 in programmed cell death: resisting ferroptosis in the cell membrane and promoting necroptosis in the nucleus of THP-1 cells.Mol Med. 2024 Jul 15;30(1):102. doi: 10.1186/s10020-024-00861-4. Mol Med. 2024. PMID: 39009982 Free PMC article.
-
Nanocages displaying SIRP gamma clusters combined with prophagocytic stimulus of phagocytes potentiate anti-tumor immunity.Cancer Gene Ther. 2021 Sep;28(9):960-970. doi: 10.1038/s41417-021-00372-y. Epub 2021 Aug 4. Cancer Gene Ther. 2021. PMID: 34349240
-
High activity and low toxicity of a novel CD71-targeting nanotherapeutic named The-0504 on preclinical models of several human aggressive tumors.J Exp Clin Cancer Res. 2021 Feb 10;40(1):63. doi: 10.1186/s13046-021-01851-8. J Exp Clin Cancer Res. 2021. PMID: 33568214 Free PMC article.
-
Comprehensive analysis of ferritin subunits expression and positive correlations with tumor-associated macrophages and T regulatory cells infiltration in most solid tumors.Aging (Albany NY). 2021 Apr 16;13(8):11491-11506. doi: 10.18632/aging.202841. Epub 2021 Apr 16. Aging (Albany NY). 2021. PMID: 33864445 Free PMC article.
-
Ferritin in cancer therapy: A pleiotropic tumoraffin nanocage-based transport.Cancer Med. 2023 May;12(10):11570-11588. doi: 10.1002/cam4.5778. Epub 2023 Mar 31. Cancer Med. 2023. PMID: 36999977 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

