ESC-sEVs Rejuvenate Aging Hippocampal NSCs by Transferring SMADs to Regulate the MYT1-Egln3-Sirt1 Axis
- PMID: 33038325
- PMCID: PMC7791087
- DOI: 10.1016/j.ymthe.2020.09.037
ESC-sEVs Rejuvenate Aging Hippocampal NSCs by Transferring SMADs to Regulate the MYT1-Egln3-Sirt1 Axis
Abstract
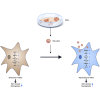
Tissue stem cell senescence leads to stem cell exhaustion, which results in tissue homeostasis imbalance and a decline in regeneration capacity. However, whether neural stem cell (NSC) senescence occurs and causes neurogenesis reduction during aging is unknown. In this study, mice at different ages were used to detect age-related hippocampal NSC (H-NSC) senescence, as well as the function and mechanism of embryonic stem cell-derived small extracellular vesicles (ESC-sEVs) in rejuvenating H-NSC senescence. We found a progressive cognitive impairment, as well as age-related H-NSC senescence, in mice. ESC-sEV treatment significantly alleviated H-NSC senescence, recovered compromised self-renewal and neurogenesis capacities, and reversed cognitive impairment. Transcriptome analysis revealed that myelin transcription factor 1 (MYT1) is downregulated in senescent H-NSCs but upregulated by ESC-sEV treatment. In addition, knockdown of MYT1 in young H-NSCs accelerated age-related phenotypes and impaired proliferation and differentiation capacities. Mechanistically, ESC-sEVs rejuvenated senescent H-NSCs partly by transferring SMAD family members 4 (SMAD4) and 5 (SMAD5) to activate MYT1, which downregulated egl-9 family hypoxia inducible factor 3 (Egln3), followed by activation of hypoxia inducible factor 2 subunit α (HIF-2α), nicotinamide phosphoribosyl transferase (NAMPT), and sirtuin 1 (Sirt1) successively. Taken together, our results indicated that H-NSC senescence caused cellular exhaustion, neurogenesis reduction, and cognitive impairment during aging, which can be reversed by ESC-sEVs. Thus, ESC-sEVs may be promising therapeutic candidates for age-related diseases.
Keywords: ESC-sEVs; MYT1; aging; hippocampal NSCs; senescence.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
ESC-sEVs Rejuvenate Senescent Hippocampal NSCs by Activating Lysosomes to Improve Cognitive Dysfunction in Vascular Dementia.Adv Sci (Weinh). 2020 Mar 20;7(10):1903330. doi: 10.1002/advs.201903330. eCollection 2020 May. Adv Sci (Weinh). 2020. PMID: 32440476 Free PMC article.
-
Inducible Pluripotent Stem Cell-Derived Small Extracellular Vesicles Rejuvenate Senescent Blood-Brain Barrier to Protect against Ischemic Stroke in Aged Mice.ACS Nano. 2023 Jan 10;17(1):775-789. doi: 10.1021/acsnano.2c10824. Epub 2022 Dec 23. ACS Nano. 2023. PMID: 36562422
-
Induced Mesenchymal Stem Cells-Small Extracellular Vesicles Alleviate Post-stroke Cognitive Impairment by Rejuvenating Senescence of Neural Stem Cells.J Mol Neurosci. 2024 Mar 13;74(1):29. doi: 10.1007/s12031-024-02191-w. J Mol Neurosci. 2024. PMID: 38478127
-
From Youthful Vigor to Aging Decline: Unravelling the Intrinsic and Extrinsic Determinants of Hippocampal Neural Stem Cell Aging.Cells. 2023 Aug 17;12(16):2086. doi: 10.3390/cells12162086. Cells. 2023. PMID: 37626896 Free PMC article. Review.
-
Mechanisms of enhanced quiescence in neural stem cell aging.Mech Ageing Dev. 2020 Oct;191:111323. doi: 10.1016/j.mad.2020.111323. Epub 2020 Aug 8. Mech Ageing Dev. 2020. PMID: 32781077 Free PMC article. Review.
Cited by
-
Extracellular Vesicle-Based Therapeutics in Neurological Disorders.Pharmaceutics. 2022 Nov 30;14(12):2652. doi: 10.3390/pharmaceutics14122652. Pharmaceutics. 2022. PMID: 36559145 Free PMC article. Review.
-
Roles of extracellular vesicles in the aging microenvironment and age-related diseases.J Extracell Vesicles. 2021 Oct;10(12):e12154. doi: 10.1002/jev2.12154. J Extracell Vesicles. 2021. PMID: 34609061 Free PMC article. Review.
-
Extracellular vesicle biogenesis of three-dimensional human pluripotent stem cells in a novel Vertical-Wheel bioreactor.J Extracell Biol. 2024 Jan 9;3(1):e133. doi: 10.1002/jex2.133. eCollection 2024 Jan. J Extracell Biol. 2024. PMID: 38938678 Free PMC article.
-
iPSC-sEVs alleviate microglia senescence to protect against ischemic stroke in aged mice.Mater Today Bio. 2023 Mar 4;19:100600. doi: 10.1016/j.mtbio.2023.100600. eCollection 2023 Apr. Mater Today Bio. 2023. PMID: 36936398 Free PMC article.
-
Young small extracellular vesicles rejuvenate replicative senescence by remodeling Drp1 translocation-mediated mitochondrial dynamics.J Nanobiotechnology. 2024 Sep 5;22(1):543. doi: 10.1186/s12951-024-02818-5. J Nanobiotechnology. 2024. PMID: 39238005 Free PMC article.
References
-
- Bouab M., Paliouras G.N., Aumont A., Forest-Bérard K., Fernandes K.J. Aging of the subventricular zone neural stem cell niche: evidence for quiescence-associated changes between early and mid-adulthood. Neuroscience. 2011;173:135–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

