A severe leakage of intermediates to shunt products in acarbose biosynthesis
- PMID: 32193369
- PMCID: PMC7081202
- DOI: 10.1038/s41467-020-15234-8
A severe leakage of intermediates to shunt products in acarbose biosynthesis
Abstract
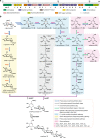
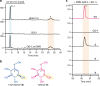
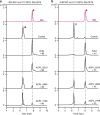
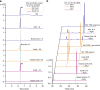
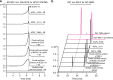
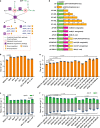
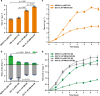
The α-glucosidase inhibitor acarbose, produced by Actinoplanes sp. SE50/110, is a well-known drug for the treatment of type 2 diabetes mellitus. However, the largely unexplored biosynthetic mechanism of this compound has impeded further titer improvement. Herein, we uncover that 1-epi-valienol and valienol, accumulated in the fermentation broth at a strikingly high molar ratio to acarbose, are shunt products that are not directly involved in acarbose biosynthesis. Additionally, we find that inefficient biosynthesis of the amino-deoxyhexose moiety plays a role in the formation of these shunt products. Therefore, strategies to minimize the flux to the shunt products and to maximize the supply of the amino-deoxyhexose moiety are implemented, which increase the acarbose titer by 1.2-fold to 7.4 g L-1. This work provides insights into the biosynthesis of the C7-cyclitol moiety and highlights the importance of assessing shunt product accumulation when seeking to improve the titer of microbial pharmaceutical products.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Identification of a 1-epi-valienol 7-kinase activity in the producer of acarbose, Actinoplanes sp. SE50/110.FEBS Lett. 2003 Apr 10;540(1-3):53-7. doi: 10.1016/s0014-5793(03)00222-9. FEBS Lett. 2003. PMID: 12681482
-
Biosynthesis of the C(7)-cyclitol moiety of acarbose in Actinoplanes species SE50/110. 7-O-phosphorylation of the initial cyclitol precursor leads to proposal of a new biosynthetic pathway.J Biol Chem. 2002 Jun 21;277(25):22853-62. doi: 10.1074/jbc.M202375200. Epub 2002 Apr 5. J Biol Chem. 2002. PMID: 11937512
-
The complete genome sequence of the acarbose producer Actinoplanes sp. SE50/110.BMC Genomics. 2012 Mar 23;13:112. doi: 10.1186/1471-2164-13-112. BMC Genomics. 2012. PMID: 22443545 Free PMC article.
-
Biotechnology and molecular biology of the alpha-glucosidase inhibitor acarbose.Appl Microbiol Biotechnol. 2004 Feb;63(6):613-25. doi: 10.1007/s00253-003-1477-2. Epub 2003 Dec 11. Appl Microbiol Biotechnol. 2004. PMID: 14669056 Review.
-
[Biosynthesis and regulatory mechanism of acarbose and its structural analogs: a review].Sheng Wu Gong Cheng Xue Bao. 2022 Feb 25;38(2):605-619. doi: 10.13345/j.cjb.210248. Sheng Wu Gong Cheng Xue Bao. 2022. PMID: 35234385 Review. Chinese.
Cited by
-
Applications of synthetic biology in medical and pharmaceutical fields.Signal Transduct Target Ther. 2023 May 11;8(1):199. doi: 10.1038/s41392-023-01440-5. Signal Transduct Target Ther. 2023. PMID: 37169742 Free PMC article. Review.
-
Identification and bioactivity evaluation of secondary metabolites from Antarctic-derived Penicillium chrysogenum CCTCC M 2020019.RSC Adv. 2020 Jun 2;10(35):20738-20744. doi: 10.1039/d0ra03529g. eCollection 2020 May 27. RSC Adv. 2020. PMID: 35517746 Free PMC article.
-
Complete biosynthetic pathway to the antidiabetic drug acarbose.Nat Commun. 2022 Jun 15;13(1):3455. doi: 10.1038/s41467-022-31232-4. Nat Commun. 2022. PMID: 35705566 Free PMC article.
-
PAS domain containing regulator SLCG_7083 involved in morphological development and glucose utilization in Streptomyces lincolnensis.Microb Cell Fact. 2023 Dec 13;22(1):257. doi: 10.1186/s12934-023-02263-3. Microb Cell Fact. 2023. PMID: 38093313 Free PMC article.
-
Enhancement of acarbose production by genetic engineering and fed-batch fermentation strategy in Actinoplanes sp. SIPI12-34.Microb Cell Fact. 2022 Nov 23;21(1):240. doi: 10.1186/s12934-022-01969-0. Microb Cell Fact. 2022. PMID: 36419063 Free PMC article.
References
-
- Asamizu S. Biosynthesis of nitrogen-containing natural products, C7N aminocyclitols and bis-indoles, from actinomycetes. J. Agric. Chem. Soc. Jpn. 2017;81:871–881. - PubMed
-
- Ahr HJ, et al. Pharmacokinetics of acarbose. Part I: absorption, concentration in plasma, metabolism and excretion after single administration of [14C]acarbose to rats, dogs and man. Arzneimittelforschung. 1989;39:1254–1260. - PubMed
-
- Juergen, B., Michael, S. & Ulrich, S. Osmotically controlled fermentation process for the preparation of acarbose. US patent 6,130,072 (2000).
-
- Feng ZH, Wang YS, Zheng YG. A new microtiter plate-based screening method for microorganisms producing α-amylase inhibitors. Biotechnol. Bioproc. E. 2011;16:894–900. doi: 10.1007/s12257-011-0033-7. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

