Myofibroblast induces hepatocyte-to-ductal metaplasia via laminin-ɑvβ6 integrin in liver fibrosis
- PMID: 32251270
- PMCID: PMC7090046
- DOI: 10.1038/s41419-020-2372-9
Myofibroblast induces hepatocyte-to-ductal metaplasia via laminin-ɑvβ6 integrin in liver fibrosis
Abstract
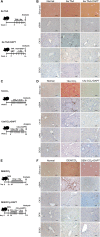
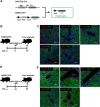
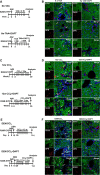
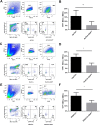
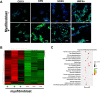
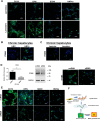
Hepatocytes undergo the metaplasia into ductal biliary epithelial cells (BECs) in response to chronic injury, and subsequently contribute to liver regeneration. The mechanism underlying hepatocyte-to-ductal metaplasia has not been explored until now. In mouse models of liver fibrosis, a florid BEC response was observed in fibrotic liver, and the depletion of myofibroblasts attenuated BEC expansion remarkably. Then, in hepatocyte fate-tracing mouse model, we demonstrated the conversion of mature hepatocytes into ductal BECs in fibrotic liver, and the depletion of myofibroblasts diminished the hepatocyte-to-ductal metaplasia. Finally, the mechanism underlying the metaplasia was investigated. Myofibroblasts secreted laminin-rich extracellular matrix, and then laminin induced hepatocyte-to-ductal metaplasia through ɑvβ6 integrin. Therefore, our results demonstrated myofibroblasts induce the conversion of mature hepatocytes into ductal BECs through laminin-ɑvβ6 integrin, which reveals that the strategy improve regeneration in fibrotic liver through the modification of specific microenvironment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Hepatocyte-Specific β-Catenin Deletion During Severe Liver Injury Provokes Cholangiocytes to Differentiate Into Hepatocytes.Hepatology. 2019 Feb;69(2):742-759. doi: 10.1002/hep.30270. Epub 2019 Jan 4. Hepatology. 2019. PMID: 30215850 Free PMC article.
-
Contribution of Mature Hepatocytes to Biliary Regeneration in Rats with Acute and Chronic Biliary Injury.PLoS One. 2015 Aug 26;10(8):e0134327. doi: 10.1371/journal.pone.0134327. eCollection 2015. PLoS One. 2015. PMID: 26308208 Free PMC article.
-
Expression of pro-fibrotic and anti-fibrotic molecules in dimethylnitrosamine-induced hepatic fibrosis.Pathol Res Pract. 2017 Jan;213(1):58-65. doi: 10.1016/j.prp.2016.11.004. Epub 2016 Nov 11. Pathol Res Pract. 2017. PMID: 27894619
-
Epithelial-mesenchymal interactions in fibrosis and repair. Transforming growth factor-β activation by epithelial cells and fibroblasts.Ann Am Thorac Soc. 2015 Mar;12 Suppl 1(Suppl 1):S21-3. doi: 10.1513/AnnalsATS.201406-245MG. Ann Am Thorac Soc. 2015. PMID: 25830829 Free PMC article. Review.
-
New Developments on the Treatment of Liver Fibrosis.Dig Dis. 2016;34(5):589-96. doi: 10.1159/000445269. Epub 2016 Jun 22. Dig Dis. 2016. PMID: 27332862 Free PMC article. Review.
Cited by
-
Interferon-γ secreted by recruited Th1 cells in peritoneal cavity inhibits the formation of malignant ascites.Cell Death Discov. 2023 Jan 23;9(1):25. doi: 10.1038/s41420-023-01312-5. Cell Death Discov. 2023. PMID: 36690649 Free PMC article.
-
Role of Immune Cells in Biliary Repair.Front Immunol. 2022 Mar 30;13:866040. doi: 10.3389/fimmu.2022.866040. eCollection 2022. Front Immunol. 2022. PMID: 35432349 Free PMC article. Review.
-
A Dynamic Transcriptome Map of Different Tissue Microenvironment Cells Identified During Gastric Cancer Development Using Single-Cell RNA Sequencing.Front Immunol. 2021 Oct 21;12:728169. doi: 10.3389/fimmu.2021.728169. eCollection 2021. Front Immunol. 2021. PMID: 34745098 Free PMC article.
-
New insights into fibrotic signaling in hepatocellular carcinoma.Front Oncol. 2023 Nov 22;13:1196298. doi: 10.3389/fonc.2023.1196298. eCollection 2023. Front Oncol. 2023. PMID: 38074679 Free PMC article. Review.
-
Resident Fibroblast MKL1 Is Sufficient to Drive Pro-fibrogenic Response in Mice.Front Cell Dev Biol. 2022 Feb 1;9:812748. doi: 10.3389/fcell.2021.812748. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35178401 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

