Circular RNA-ZNF532 regulates diabetes-induced retinal pericyte degeneration and vascular dysfunction
- PMID: 32343678
- PMCID: PMC7324174
- DOI: 10.1172/JCI123353
Circular RNA-ZNF532 regulates diabetes-induced retinal pericyte degeneration and vascular dysfunction
Abstract
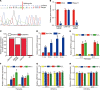
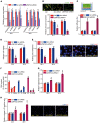
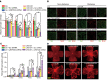
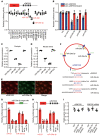
Diabetic retinopathy (DR) is the leading cause of blindness in working-age adults. Vascular pericyte degeneration is the predominant clinical manifestation of DR, yet the mechanism governing pericyte degeneration is poorly understood. Circular RNAs (circRNAs) play important roles in multiple biological processes and disease progression. Here, we investigated the role of circRNA in pericyte biology and diabetes-induced retinal vascular dysfunction. cZNF532 expression was upregulated in pericytes under diabetic stress, in the retinal vessels of a diabetic murine model, and in the vitreous humor of diabetic patients. cZNF532 silencing reduced the viability, proliferation, and differentiation of pericytes and suppressed the recruitment of pericytes toward endothelial cells in vitro. cZNF532 regulated pericyte biology by acting as a miR-29a-3p sponge and inducing increased expression of NG2, LOXL2, and CDK2. Knockdown of cZNF532 or overexpression of miR-29a-3p aggravated streptozotocin-induced retinal pericyte degeneration and vascular dysfunction. By contrast, overexpression of cZNF532 or inhibition of miR-29a-3p ameliorated human diabetic vitreous-induced retinal pericyte degeneration and vascular dysfunction. Collectively, these data identify a circRNA-mediated mechanism that coordinates pericyte biology and vascular homeostasis in DR. Induction of cZNF532 or antagonism of miR-29a-3p is an exploitable therapeutic approach for the treatment of DR.
Keywords: Diabetes; Noncoding RNAs; Ophthalmology; Retinopathy.
Conflict of interest statement
Figures

Similar articles
-
Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction.Proc Natl Acad Sci U S A. 2019 Apr 9;116(15):7455-7464. doi: 10.1073/pnas.1814874116. Epub 2019 Mar 26. Proc Natl Acad Sci U S A. 2019. PMID: 30914462 Free PMC article.
-
Long noncoding RNA MIAT regulates primary human retinal pericyte pyroptosis by modulating miR-342-3p targeting of CASP1 in diabetic retinopathy.Exp Eye Res. 2021 Jan;202:108300. doi: 10.1016/j.exer.2020.108300. Epub 2020 Oct 13. Exp Eye Res. 2021. PMID: 33065089
-
Increased Ephrin-B2 expression in pericytes contributes to retinal vascular death in rodents.Vascul Pharmacol. 2020 Aug;131:106761. doi: 10.1016/j.vph.2020.106761. Epub 2020 Jun 22. Vascul Pharmacol. 2020. PMID: 32585189 Free PMC article.
-
Targeting pericyte retention in Diabetic Retinopathy: a review.Ann Med. 2024 Dec;56(1):2398200. doi: 10.1080/07853890.2024.2398200. Epub 2024 Sep 13. Ann Med. 2024. PMID: 39268600 Free PMC article. Review.
-
miRNA, lncRNA and circRNA: Targeted Molecules Full of Therapeutic Prospects in the Development of Diabetic Retinopathy.Front Endocrinol (Lausanne). 2021 Nov 10;12:771552. doi: 10.3389/fendo.2021.771552. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34858342 Free PMC article. Review.
Cited by
-
CircRNA Is a Rising Star in Researches of Ocular Diseases.Front Cell Dev Biol. 2020 Sep 3;8:850. doi: 10.3389/fcell.2020.00850. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015046 Free PMC article. Review.
-
Identification of Potentially Functional Circular RNA/Long Noncoding RNA-MicroRNA-mRNA Regulatory Networks Associated with Vascular Injury in Type 2 Diabetes Mellitus by Integrated Microarray Analysis.J Diabetes Res. 2023 Mar 10;2023:3720602. doi: 10.1155/2023/3720602. eCollection 2023. J Diabetes Res. 2023. PMID: 36937538 Free PMC article.
-
Lipotoxicity-induced circGlis3 impairs beta cell function and is transmitted by exosomes to promote islet endothelial cell dysfunction.Diabetologia. 2022 Jan;65(1):188-205. doi: 10.1007/s00125-021-05591-4. Epub 2021 Nov 9. Diabetologia. 2022. PMID: 34751796
-
Circular RNA HMGCS1 sponges MIR4521 to aggravate type 2 diabetes-induced vascular endothelial dysfunction.Elife. 2024 Sep 5;13:RP97267. doi: 10.7554/eLife.97267. Elife. 2024. PMID: 39235443 Free PMC article.
-
Circular RNAs: Novel target of diabetic retinopathy.Rev Endocr Metab Disord. 2021 Jun;22(2):205-216. doi: 10.1007/s11154-021-09646-0. Epub 2021 Mar 24. Rev Endocr Metab Disord. 2021. PMID: 33761053 Review.

