Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer
- PMID: 32939032
- PMCID: PMC8027668
- DOI: 10.1038/s41423-020-0515-7
Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer
Abstract
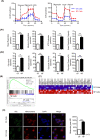
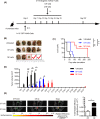
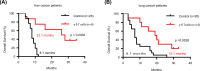
Vγ9Vδ2 T cells are promising candidates for cellular tumor immunotherapy. Due to their HLA-independent mode of action, allogeneic Vγ9Vδ2 T cells can be considered for clinical application. To apply allogeneic Vγ9Vδ2 T cells in adoptive immunotherapy, the methodology used to obtain adequate cell numbers with optimal effector function in vitro needs to be optimized, and clinical safety and efficacy also need to be proven. Therefore, we developed a novel formula to improve the expansion of peripheral γδ T cells from healthy donors. Then, we used a humanized mouse model to validate the therapeutic efficacy of expanded γδ T cells in vivo; furthermore, the expanded γδ T cells were adoptively transferred into late-stage liver and lung cancer patients. We found that the expanded cells possessed significantly improved immune effector functions, including proliferation, differentiation, and cancer cell killing, both in vitro and in the humanized mouse model. Furthermore, a phase I clinical trial in 132 late-stage cancer patients with a total of 414 cell infusions unequivocally validated the clinical safety of allogeneic Vγ9Vδ2 T cells. Among these 132 patients, 8 liver cancer patients and 10 lung cancer patients who received ≥5 cell infusions showed greatly prolonged survival, which preliminarily verified the efficacy of allogeneic Vγ9Vδ2 T-cell therapy. Our clinical studies underscore the safety and efficacy of allogeneic Vγ9Vδ2 T-cell immunotherapy, which will inspire further clinical investigations and eventually benefit cancer patients.
Keywords: Allogeneic γδ T cells; Cell therapy; Humanized mice; Liver cancer; Lung cancer; New expansion formula.
Conflict of interest statement
A PCT patent (PCT/CN2019/075491) for the new formula for γδ T-cell expansion was filed. D.K. is a member of the Scientific Advisory Boards of Incysus, Imcheck, and Lava Therapeutics.
Figures


Similar articles
-
Vgamma9Vdelta2 T cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas.Cancer Immunol Immunother. 2008 Apr;57(4):531-9. doi: 10.1007/s00262-007-0391-3. Epub 2007 Sep 1. Cancer Immunol Immunother. 2008. PMID: 17764010 Free PMC article.
-
Systemic β-Adrenergic Receptor Activation Augments the ex vivo Expansion and Anti-Tumor Activity of Vγ9Vδ2 T-Cells.Front Immunol. 2020 Jan 24;10:3082. doi: 10.3389/fimmu.2019.03082. eCollection 2019. Front Immunol. 2020. PMID: 32038628 Free PMC article.
-
Adoptive transfer of ex vivo expanded Vγ9Vδ2 T cells in combination with zoledronic acid inhibits cancer growth and limits osteolysis in a murine model of osteolytic breast cancer.Cancer Lett. 2017 Feb 1;386:141-150. doi: 10.1016/j.canlet.2016.11.013. Epub 2016 Nov 16. Cancer Lett. 2017. PMID: 27865798 Free PMC article.
-
Improving the Efficiency of Vγ9Vδ2 T-Cell Immunotherapy in Cancer.Front Immunol. 2018 Apr 19;9:800. doi: 10.3389/fimmu.2018.00800. eCollection 2018. Front Immunol. 2018. PMID: 29725332 Free PMC article. Review.
-
γδ T cells in cancer immunotherapy.Oncotarget. 2017 Jan 31;8(5):8900-8909. doi: 10.18632/oncotarget.13051. Oncotarget. 2017. PMID: 27823972 Free PMC article. Review.
Cited by
-
The genomic landscape of the immune system in lung cancer: present insights and continuing investigations.Front Genet. 2024 Jun 25;15:1414487. doi: 10.3389/fgene.2024.1414487. eCollection 2024. Front Genet. 2024. PMID: 38983267 Free PMC article. Review.
-
Reducing farnesyl diphosphate synthase levels activates Vγ9Vδ2 T cells and improves tumor suppression in murine xenograft cancer models.Front Immunol. 2022 Oct 5;13:1012051. doi: 10.3389/fimmu.2022.1012051. eCollection 2022. Front Immunol. 2022. PMID: 36275712 Free PMC article.
-
Immune Evasion Through Human Leukocyte Antigen Implications and Its Impact on Targeted Therapy.Cureus. 2024 Jan 22;16(1):e52737. doi: 10.7759/cureus.52737. eCollection 2024 Jan. Cureus. 2024. PMID: 38384647 Free PMC article. Review.
-
High TRGV 9 Subfamily Expression Marks an Improved Overall Survival in Patients With Acute Myeloid Leukemia.Front Immunol. 2022 Feb 10;13:823352. doi: 10.3389/fimmu.2022.823352. eCollection 2022. Front Immunol. 2022. PMID: 35222403 Free PMC article.
-
Vdelta1 T cells are more resistant than Vdelta2 T cells to the immunosuppressive properties of galectin-3.Front Immunol. 2024 Jan 8;14:1286097. doi: 10.3389/fimmu.2023.1286097. eCollection 2023. Front Immunol. 2024. PMID: 38259448 Free PMC article.
References
-
- Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. - PubMed
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–86. - PubMed
-
- Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 2018;15:31–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

