microRNA-630 Regulates Underglycosylated IgA1 Production in the Tonsils by Targeting TLR4 in IgA Nephropathy
- PMID: 33324395
- PMCID: PMC7725902
- DOI: 10.3389/fimmu.2020.563699
microRNA-630 Regulates Underglycosylated IgA1 Production in the Tonsils by Targeting TLR4 in IgA Nephropathy
Abstract
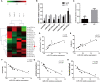
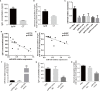
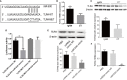
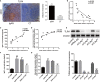
IgA nephropathy (IgAN) is the most common primary glomerular disease. The characteristic pathology involves immune complexes formed by the deposition of IgA1 and underglycosylated IgA1 aggregates in the mesangial area, which may be accompanied by the deposition of IgG and/or IgM and complement components. However, the molecular mechanisms of IgAN remain unclear. In the present study, microarray analysis showed that the expression of microRNA-630 (miR-630) was significantly reduced in palatal tonsils from IgAN patients compared with chronic tonsillitis. Additionally, bioinformatic analysis showed that Toll-like receptor 4 (TLR4) was the predicted target gene of miR-630 and was regulated by miR-630. When miR-630 was overexpressed in palatal tonsil mononuclear cells from IgAN patients, the expression of TLR4 was reduced and the content of IgA1 in the cell culture supernatant was decreased, and the level of galactosylation in the IgA1 hinge region was increased. Moreover, immunohistochemical analysis showed that the expression of TLR4 in IgAN patients was significantly increased. After knocking down the expression of TLR4, both the concentration of IgA1 and the binding force of IgA1 with broad bean lectin were significantly reduced in IgAN. Furthermore, the mechanism study demonstrated that TLR4 might regulate the expression of IL-1β and IL-8 through NF-κB signaling pathway to modulate the concentration of IgA1 and the glycosylation level of IgA1. This interesting finding may offer new insight into the molecular mechanism of IgAN.
Keywords: IgA nephropathy; TLR4; microRNA-630; palatal tonsils; underglycosylated IgA1.
Copyright © 2020 Liu, Ye, Yan, Peng, He and Peng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer H. L. declared a shared affiliation with the authors to the handling editor at time of review.
Figures
Similar articles
-
Activation of the interleukin-4/signal transducer and activator of transcription 6 signaling pathway and homeodomain-interacting protein kinase 2 production by tonsillar mononuclear cells in IgA nephropathy.Am J Nephrol. 2013;38(4):321-32. doi: 10.1159/000355393. Epub 2013 Oct 4. Am J Nephrol. 2013. PMID: 24107646
-
Loss of the Golgi Matrix Protein 130 Cause Aberrant IgA1 Glycosylation in IgA Nephropathy.Am J Nephrol. 2019;49(4):307-316. doi: 10.1159/000499110. Epub 2019 Mar 27. Am J Nephrol. 2019. PMID: 30917363
-
IgA nephropathy and tonsils--an approach from the structure of IgA1 produced by tonsillar lymphocytes.Acta Otolaryngol Suppl. 2004 Dec;(555):28-31. doi: 10.1080/03655230410003314. Acta Otolaryngol Suppl. 2004. PMID: 15768794
-
IgA nephropathy and aberrant glycosylation of tonsillar, serum and glomerular IgA1.Adv Otorhinolaryngol. 2011;72:68-70. doi: 10.1159/000324609. Epub 2011 Aug 18. Adv Otorhinolaryngol. 2011. PMID: 21865693 Review.
-
IgA production and tonsillar focal infection in IgA nephropathy.J Clin Exp Hematop. 2012;52(3):161-70. doi: 10.3960/jslrt.52.161. J Clin Exp Hematop. 2012. PMID: 23269075 Review.
Cited by
-
IgA nephropathy: gut microbiome regulates the production of hypoglycosilated IgA1 via the TLR4 signaling pathway.Nephrol Dial Transplant. 2024 Sep 27;39(10):1624-1641. doi: 10.1093/ndt/gfae052. Nephrol Dial Transplant. 2024. PMID: 38402460 Free PMC article.
-
Ferroptosis-Related Genes in IgA Nephropathy: Screening for Potential Targets of the Mechanism.Int J Genomics. 2024 Aug 14;2024:8851124. doi: 10.1155/2024/8851124. eCollection 2024. Int J Genomics. 2024. PMID: 39171207 Free PMC article.
-
MicroRNA-630 alleviates inflammatory reactions in rats with diabetic kidney disease by targeting toll-like receptor 4.World J Diabetes. 2024 Mar 15;15(3):488-501. doi: 10.4239/wjd.v15.i3.488. World J Diabetes. 2024. PMID: 38591087 Free PMC article.
-
Aberrantly Glycosylated IgA1 in IgA Nephropathy: What We Know and What We Don't Know.J Clin Med. 2021 Aug 5;10(16):3467. doi: 10.3390/jcm10163467. J Clin Med. 2021. PMID: 34441764 Free PMC article. Review.
-
Title IgA Nephropathy and Oral Bacterial Species Related to Dental Caries and Periodontitis.Int J Mol Sci. 2022 Jan 10;23(2):725. doi: 10.3390/ijms23020725. Int J Mol Sci. 2022. PMID: 35054910 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

